- View Mobile Number
iirtdelhi@gmail.com
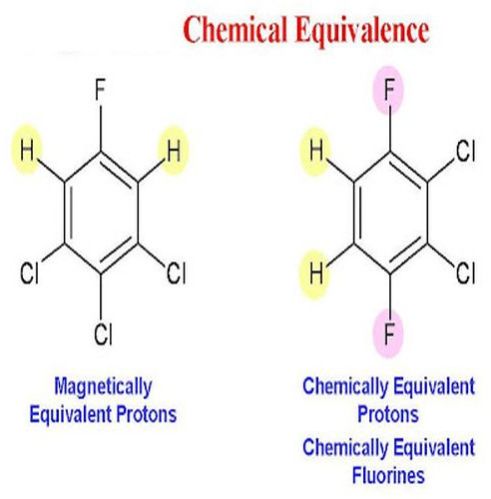
Chemical equivalence refers to the evaluation and confirmation that two or more chemical substances or formulations possess the same chemical identity, composition, and potency. This assessment ensures that the substances are effectively interchangeable for their intended application without compromising safety, quality, or performance.
Chemical equivalence is particularly important in the development of generic drugs, medical devices, and pharmaceutical formulations, where it must be demonstrated that the active ingredient in a test product matches that of the reference product. This includes verification of molecular structure, concentration, and purity levels.
Establishing chemical equivalence supports regulatory approvals, aids in quality control, and helps maintain therapeutic consistency across different products. It also ensures patients receive the same clinical benefits, regardless of the product source. Analytical methods such as spectroscopy, chromatography, and potency assays are commonly used to confirm chemical equivalence in both research and manufacturing settings.