- View Mobile Number
iirtdelhi@gmail.com
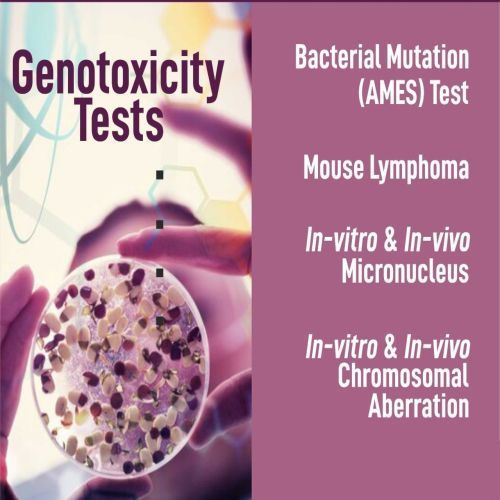
Genotoxicity testing is conducted to assess whether materials used in medical devices—or their extracts—have the potential to cause genetic damage. This damage may include gene mutations, chromosomal aberrations, or DNA strand breaks, all of which could lead to serious health effects such as cancer or heritable genetic disorders. These tests are an essential part of the biological safety evaluation of medical devices, especially those intended for long-term or internal use.
The international standard ISO 10993-3 outlines the recommended procedures and requirements for genotoxicity testing as part of a comprehensive risk assessment. It helps ensure that device materials do not pose a genetic hazard to users. Commonly used test methods include the Ames test (for gene mutations), the in vitro chromosomal aberration test, and the micronucleus assay, among others. These evaluations support regulatory submissions and ensure that medical devices meet global safety standards.